At normal everyday temperatures, all materials have some amount of electrical resistance—that is, a current of electrons cannot flow through without losing energy. Superconductivity is a rare and not well understood property of certain materials to conduct electricity with perfect efficiency when cooled under a certain (usually extremely low) critical temperature (Tc). Scientists have been able to find certain cuprates (crystal-structured layers of copper & oxygen) that superconduct at a maximum of 100 kelvin, a critical temperature that is extremely high compared to other superconductors. In theory, creating or finding a substance that can superconduct in our ~300 kelvin (80 degrees fahrenheit) everyday world would drastically reduce energy consumption by enabling lossless power lines and even pave the way for levitating vehicles and other hyper-efficient devices.
Scientists do have a base understanding of how superconductivity works in most materials.
An electron moving through a conductor’s lattice of atoms repels the electron clouds of nearby atoms, creating a partially positively charged area that travels with the electron called a Phonon.
This positively charged area can attract another electron, and together, these indirectly attracted electrons travel in what’s called a Cooper pair. Because the Cooper pair is more stable than a single electron, it experiences less resistance and allows for superconductivity. However, this can only happen below Tc because above the threshold, the increased thermal energy and vibration is too high to allow sustained existence of Cooper pairs. After decades of research, we still aren’t sure how exactly to tweak substances to encourage the formation of bosons. However, much progress has been made recently in the realm of condensed matter physics that holds promise in uncovering these mysteries.
2D Materials & Molybdenum disulfide
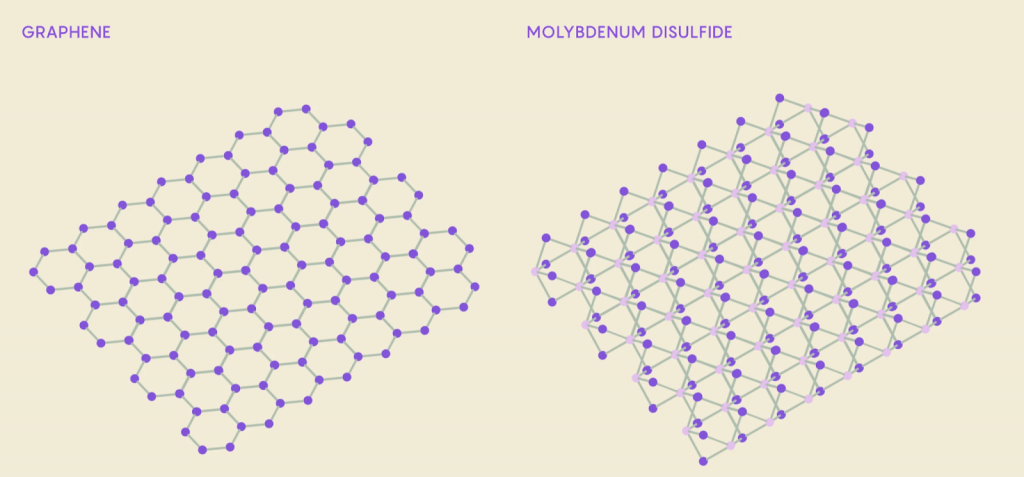
Molybdenum disulfide (MD) is a solid transition metal dichalcogenide (TMD) that has the rare ability to become a “2D material”—because it can be separated into strong 1-atom-thick sheets. It has been instrumental in the study of superconductivity. What’s exciting is that it is one of few 2D materials that are semiconductors, a material that can start and stop its flow of electrons, and could therefore serve as a transistor in electronics.
An incredible advantage to working with 2D materials is their stackability; when stacking 2D sheets, the atoms of each sheet will practically layer right on top of each other, effectively merging the sheets into one substance. Different combinations of different 2D materials sometimes have the ability to greatly enhance the properties of the material. As a result, researchers have a much more efficient way to try and discover new materials by messing around with these atomic “Lego blocks.”
Excitons
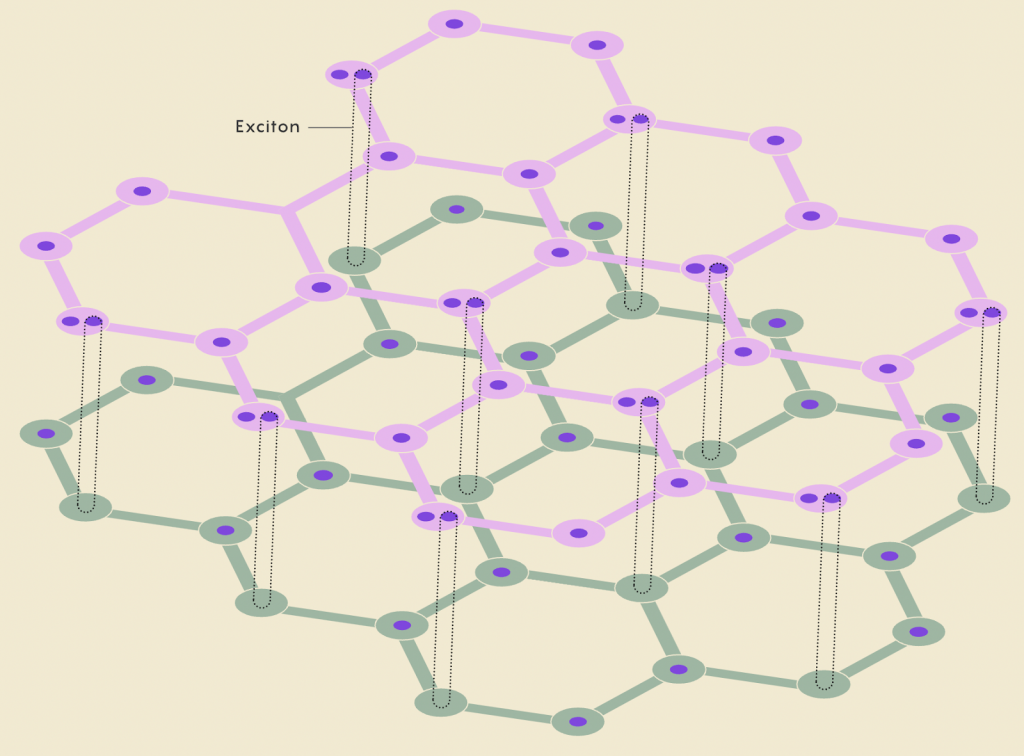
Electrons are not the only entities that move through stacked layers of 2D TMDs; electron “holes” (created when electrons jump to atoms in other layers) can move through the TMDs like real positively charged particles. These positive holes can temporarily attract a negative electron in an adjacent layer, creating an exciton. In most materials, excitons exist very temporarily, since the electrons have a tendency to jump back into their “hole.” However, excitons were found to be hundreds of times longer-lasting in TMDs than in typical 3D materials, since the electrons have trouble jumping between the layers.
Because of this, excitons may offer a roundabout way to achieve room-temperature superconductivity. Excitons act similarly to electron-electron pairs since the electron-hole pairs are also bosons; this lets them “condense” into a shared quantum state known as a Bose-Einstein condensate, which can achieve superconductivity. Although excitons form much more easily than repulsive electron pairs, a big challenge has been getting the electrically neutral pairs to flow in a current at a similar Tc to cuprates.
A current proposal for solving this problem is making the excitons flow by applying an electric field oriented in a way that encourages both electrons and holes to move in the same direction.
Many scientists believe that we are on the cusp of getting excitons to flow at 100 kelvins, which is still much lower than everyday temperatures but great progress nonetheless.
Moiré Superlattice & Artificial Atoms
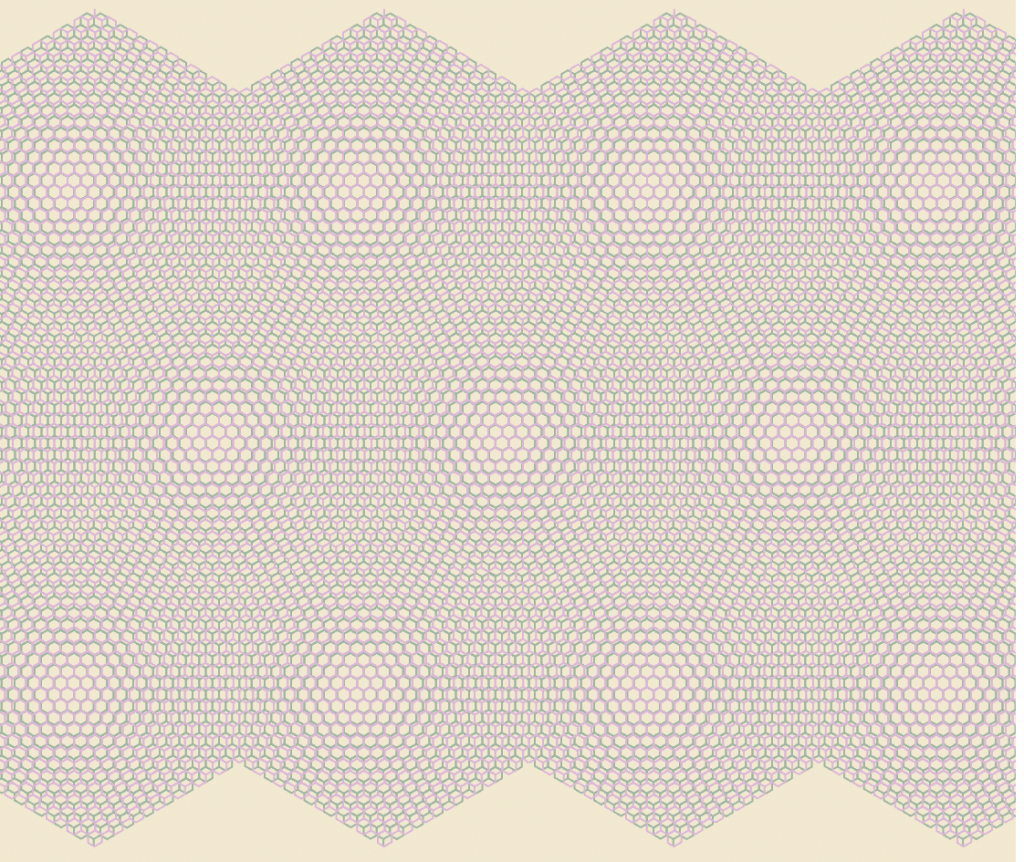
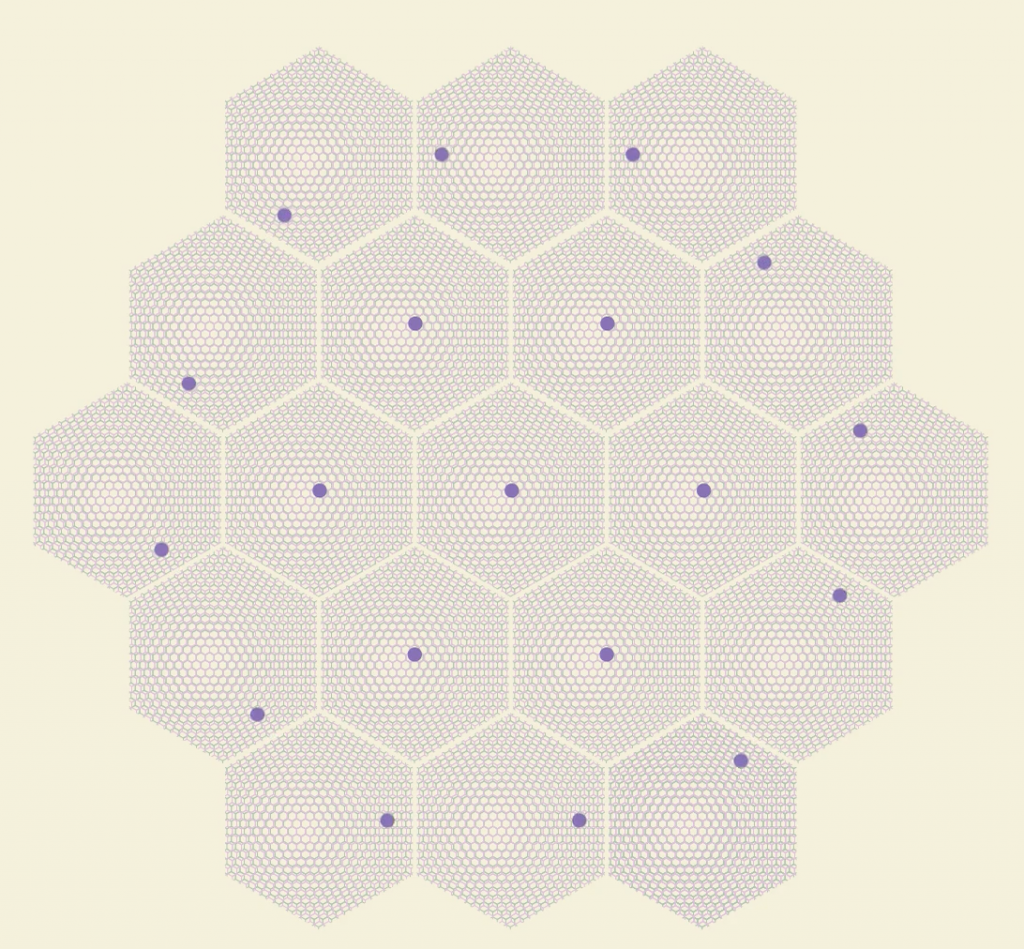
moiré superlattice–each large hexagon is a “supercell” that acts like a giant atom
By stacking different TMDs with hexagonal lattices of different sizes, the slightly larger hexagons over smaller hexagons creates a repeating pattern of large “supercells” called a moiré superlattice. Normally electrons in 2D material race around too quickly to “notice” each other, but the unique superlative structure makes the electrons slow down and sense the repulsion of their neighbors. Once this happens, the electrons take on a variety of exotic quantum states, and as a result, the TMD material gains strong superconductivity. (However, little is known about why this actually happens). Incredibly, in the superlattice, the electrons become oblivious to the individual atoms and behave like the supercells are giant atoms.
Electrons often move between atoms by tunneling, a “teleportation” quantum mechanical behavior. But tunneling rarely happens in a moiré lattice, since supercells are 100 times further apart than their constituent atoms. This makes TMD moiré materials ideal playgrounds for observing electron interactions.
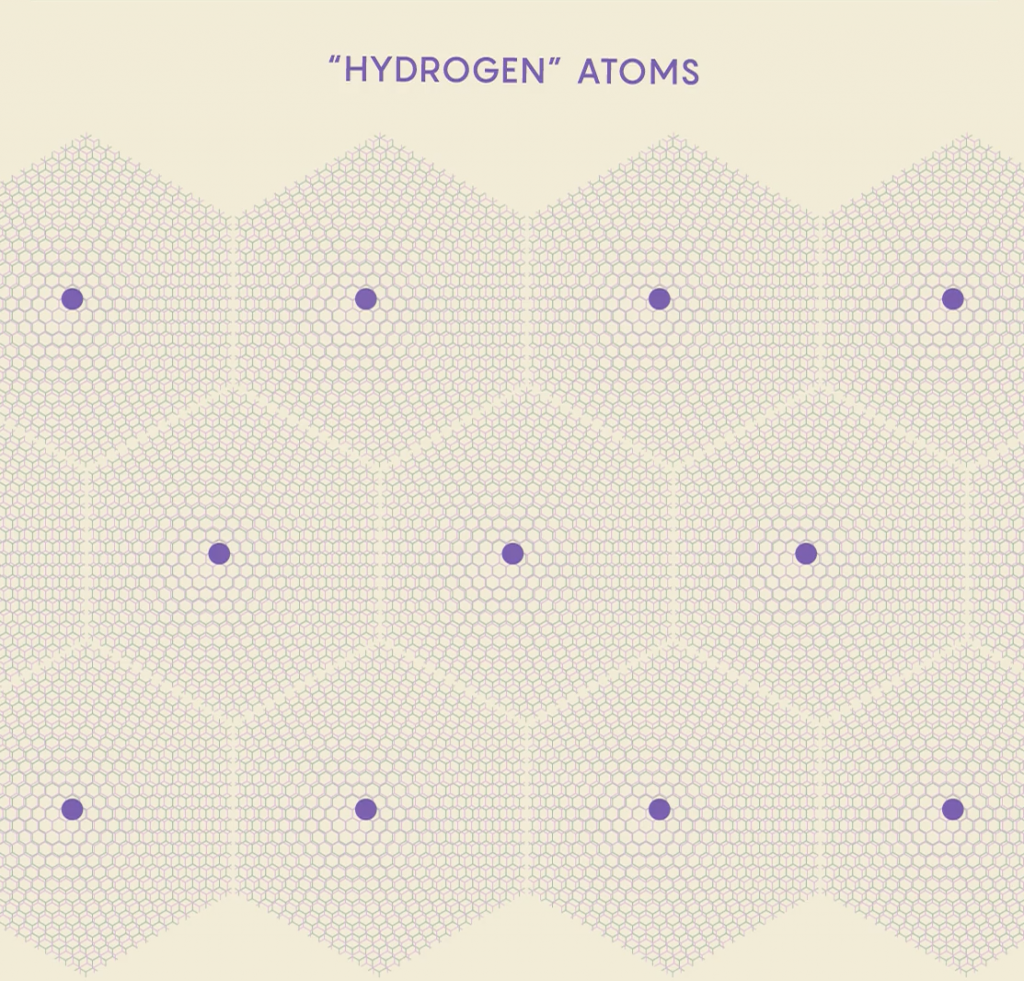


scientists have been able to reach 8 electrons per supercell, simulating “Oxygen” atoms
Scientists created “artificial atoms” by making a TMD moiré superlattice of 2D tungsten diselenide and 2D tungsten sulfide. Using electrodes to adjust the electric field passing through the TMDs, scientists adjusted the average number of electrons per supercell. Additional electrodes could also send currents through the material to create positively charged zones in the centers of the supercells that mimic “protons”. Since the cells act like giant atoms, changing electron and proton counts was similar to transforming the supercells into different elements. Adding protons also allows scientists to adjust for the tendency of electrons to tunnel to other atoms—the larger the charge of the nucleus, the stronger it holds onto its electrons.
Then scientists stumbled upon a groundbreaking discovery: some researchers accidentally created the “artificial atoms” superlattice with the top TMD layer in the wrong orientation. As they decreased the charge of the nucleus, the electrical resistance plummeted abruptly to near-superconductivity levels. Further measurements indicated that the unique structure of the lattice was making electrons on the edge of the material flow in one direction while keeping all interior electrons trapped in an insulating state. This unusual and fragile type of matter is known as a Chern insulator. Although current TMD Chern insulators can only stay together at around 5 kelvins, scientists believe that by testing different combinations of TMDs, 50-100 kelvin Chern TMDs are achievable in the near future. If this happens, superconducting nanowires in devices may become viable.
The Future
Current research is focused on developing the fundamental theory regarding the mysterious nature of superconductivity. Scientists believe that further study with artificial TMD atoms will allow them to better understand the “glue” that binds together electron pairs in superconducting cuprates (since they still are the highest temperature superconductors). Even if widespread superconductivity fails to become reality, the related research, which has spawned breakthroughs in Bose-Einstein condensates and Chern insulators, could lead to ultra-sensitive quantum sensors and enable powerful quantum computers. The future of superconductivity is uncertain, but the progress of material science has always been impossible to predict—world-changing breakthroughs could happen any day, whether it is through the lens of a new theory or a scientist accidentally putting a sheet upside down.






